| Zinc Supplementation in High School Distance Runners |
| By: Scott L. Christensen and Dr. Janet E. Steele
Originally Published in: Techniques Magazine - Provided by: USTFCCCA
Influence of zinc supplementation on three self-reported respiratory health indicators in a sample of adolescent distance runners. Purpose: Zinc supplementation was administered concurrently with 40 miles per week of aerobic distance training in adolescent male runners to isolate the influence of the supplementation on the respiratory health of the subjects. Methods: Twenty male adolescent athletes capable of voluntarily running every day at a training volume of 40 miles per week for a 10 week experimental period were studied. During the training period 10 subjects orally supplemented 15 mg of mineral zinc in tablet form every day to their normal diet. Every other day they completed a self-inventory assessment of upper respiratory tract infections (URTI) symptoms, using the Daily Analysis of Life's Demand for Athletes (DALDA). Results: Subjects who consumed zinc supplements daily did not show a significantly different number of days expressing the three key symptoms of URTI compared with non-supplemented, similarly trained, placebo group subjects (p=.46, p=.40, p=.20). Conclusion: The zinc supplemented adolescent athletes showed some positive variance in affected days of URTI symptoms, although the difference was not statistically significant. These results suggest that adolescent distance runners do not profit from 15 mg zinc supplementation to their daily diet with regard to reduction of URTI. Further studies might include a comprehensive inventory of the subject's diet to accompany a zinc supplementation study protocol of distance runners. INTRODUCTION The importance of ingesting the trace mineral zinc on a regular basis has been scientifically shown to be part of a good nutrition plan for all humans (36). Ideally, humans need to replenish 1% of their baseline zinc supply each day, with the best source being red meat from beef or lamb (40). Since the discovery in 1963 of the significance of dietary zinc in human physiology, many mechanisms responsible for the patho-physiology of zinc deficiency have become clear (36). The observed association between the increased susceptibility to immune-deficiency, infectious diseases, and low human plasma zinc concentrations led physiologists and nutritionists to speculate that there must be a link between zinc and immunity function (23). Zinc deficiency in humans is mainly due to a lack of bio-available zinc in the diet, general malnutrition or malabsorption (48). Studies indicate that specific populations of humans such as vegetarians, pregnant women, the elderly, the under-fed, and endurance athletes, particularly those who adopt a diet rich in carbohydrate but low in protein and fat, are at risk for zinc deficiency (24, 25). This may lead to loss of body weight, latent fatigue, and a compromised immune system (49). Zinc is known to be essential for all highly proliferating cells in the human body, especially the immune system (19). Low plasma zinc levels in humans impair natural killer cell activity and phagocytosis by macrophages and neutrophils (20). However, zinc is important even for the maturation and functioning of T-cells because zinc is an essential cofactor for the nonapeptidic thymus hormone thymulin, an immunity mediator (16). Thymulin is secreted by thymic epithelial cells, and requires the presence of zinc for its biological activity (5). This peptide promotes T-cell maturation, cytotoxicity, and IL-2 production, all of which are key components of the human immune system (8). Zinc has been shown to bind to thymulin in a 1:1 stoichiometry via the side chains of the amino acid asparagine and the hydroxyl groups of the two serines (13). Thymulin activity in mammals is dependent on plasma zinc concentration, so marginal changes in zinc intake or availability affect thymulin activity (8). Thymulin is detectable in the serum of zinc deficient patients but is not active (11). In addition, zinc is a major intracellular regulator of lymphocyte apoptosis (12). Lymphopenia, which is linked to zinc deficiency is due to an alteration in the production of lymphocytes, is evidenced by the loss of precursor cells via an apoptotic mechanism and is evidenced by an increased amount of glucocorticoids leading to depression of the immune system (44). The human body contains 2-4 g of zinc, and occurs in a concentration of 12-16 pmol/L in the plasma (24). Although the zinc plasma pool is very small, it is highly mobile and immunologically very important (41). Nutritional zinc requirements are difficult to deter-mine since many dietary factors affect the bioavailability of zinc, and physiological requirements of zinc vary greatly between age groups (1). However, recommended daily allowances [RDA by the U.S. National Research Council 1989] of 12-15 mg for all adolescents and 15 mg for adult males appear reasonable to achieve a normal plasma zinc level of 11-18 pmol/L (48). On the other end of the scale, it has been difficult to determine what the healthy upper limit of the daily dietary or supplemental zinc ingestion for humans (47). The World Health Organization established safe upper limits of daily zinc intake at 13 mg for infants up to one year, 23 mg for children aged 1-6 years, 32-34 mg for adolescents and up to 50 mg for adults (48). However, it appears that these recommendations are based on short-term trials and they do not take into account the possible long-term hazards of zinc supplementation (37). A literature review discussion by Favier included this statement: "Most of the authors agree in considering 50 mg of daily zinc supplementation as safe over the ages of 14 years" (9). Another study recommended a total intake of only 20 mg of zinc daily for adolescents (29). At supplemental zinc dosages of 100-300 mg per day, which would be 10 times the daily requirement, diminished lymphocyte proliferation, neutrophil chemotaxis and phagocytosis have been reported in humans (4). Studies also show that monocytes and T-cells show a different susceptibility to high zinc dosages. High zinc levels stimulate monocytes, but T-cell function is impaired at much lower zinc plasma concentration (48). This may help explain why both high and low high plasma zinc concentrations appear to suppress the immune system (6). Because of the problematic nature of establishing an adequate human zinc dosage, the 1989 RDA committee recommended that daily (chronic) ingestion of zinc supplements at not exceeding 15 mg per day without medical supervision [National Research Council 1989] (3).
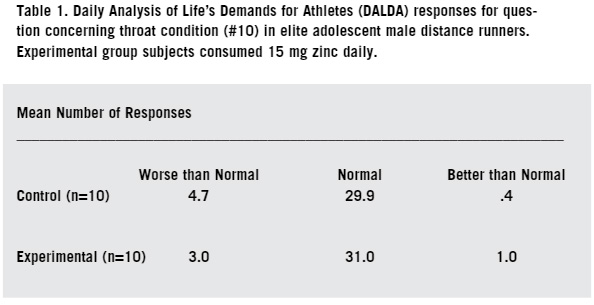
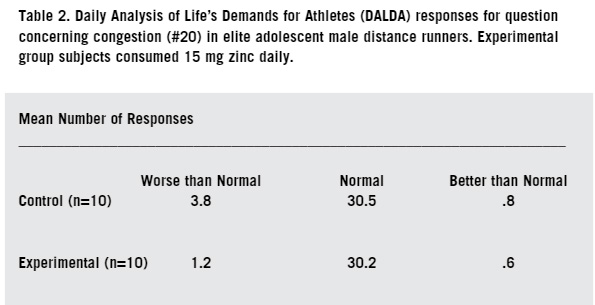
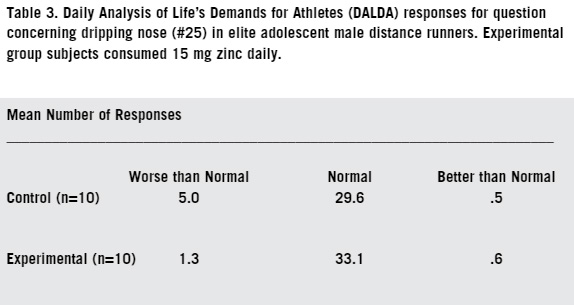
Immunosuppression in athletes involved in substantial amounts of endurance training is undoubtedly multifactorial in origin (14). Dietary deficiencies by such athletes of protein and other micro-nutrients have long been associated with immune dysfunction (21). An adequate intake of iron, zinc, and B vitamins is particularly important in proper nutrition, however there are dangers in large doses and supplementation is a real problem because many micro-nutrients when taken in large doses actually reduce human immune responses (15). To maintain immune function, endurance athletes should consume a well-balanced diet sufficient to meet their energy demands (10). An athlete exercising in a carbohydrate-depleted state experiences larger increases in circulating stress hormones and a greater perturbation of several immune deficiency symptoms (17). Endurance athletes that maintain a high carbohydrate diet to alleviate carbohydrate depletion stress usually do so at the expense of protein intake (2). Zinc is a micro nutrient that is most readily found in animal based proteins, so a diet deficient in animal proteins will lead to low levels of both zinc and iron in athletes (42). Aerobic running (training of more than 25 miles per week) is considered elite training because of the demands on the body (45). Elite training has been suggested as a link to increased susceptibility to upper respiratory tract infections [URTI] during periods of heavy training and the one to two week period following such training (32). Several researchers have reported a diminished neutrophil function in endurance athletes during periods of intense and heavy training (34). Following each bout of prolonged heavy endurance exercise, several components of the immune system appear to demonstrate suppressed function for several hours (27). Zinc is a fractional component of human sweat, and like other micronutrient components, is lost through the evaporative cooling process of the body and through exhalation especially under dry atmospheric conditions (43). Loss of zinc through sweat, coupled with a dietary deficiency of zinc due to a compromised protein intake, suggests a possible immunological link to low plasma zinc and the increased incidence of URTI that are found in some endurance runners (31, 33). A large body of epidemiological literature has examined the effects of differing amounts of intensities and exercise on a wide variety of respiratory symptoms. One published study (18), showed that athletes running approximately 30 miles per week had a significantly higher risk of self-reported URTI than runners at 15 miles per week. The running/mileage study (28) demonstrated that a single bout of intense exercise can cause serious deficiencies in immune defense when a potentially lethal virus is contracted. Another study suggests that moderate exercise of 10-15 miles per week actually improves the function of certain T-cells, but exercise in excess of 25 miles per week has a suppressive effect on T-cells (22). Martin (2009) showed symptoms of URTI were seven times higher in a self-reporting inventory among a group of elite endurance runners exercising at 50 miles per week over the group exercising at 25 miles per week. The goal of this study is to evaluate the influence of 15 mg daily dietary zinc supplementation on the respiratory health of male elite distance runners ages 16-18. While the health benefits of moderate aerobic exercise has been well established (18), intense exercise of lengthy duration is associated with depressed immune systems in both small animal models and in humans (22). Plasma zinc deficiency has been linked to a depressed immune system. The high carbohydrate/low protein diet of an endurance runner coupled with mineral loss in sweat has been shown to produce a low plasma zinc concentration (46). MATERIALS AND METHODS SUBJECTS This study was approved by the Internal Review Board (IRB) of the University of Nebraska at Kearney (protocol #120511-11). Invitation to participate in the study, youth consent form, and a parent informational letter were mailed to all 78 members of the Stillwater (MN) High School boys' cross country team. Of the 48 athletes who expressed interest in participating, 20 were chosen at random to serve as subjects. The subjects were then randomly placed into two study groups of ten each. One group served as the experimental group and was given the zinc supplement, and the other 10 subjects served as the control group. ZINC SUPPLEMENTATION The experimental subjects were each given a supply of 70 tablets of 15 mg of zinc (Nash Finch Co, #17863 34563) in a clear plastic bottle. The control (placebo) subjects were given 70 tablets each of a dextrose/green tea tablet (Glucotabs, BBI Healthcare), in an identical bottle. Both tablet supplies looked similar and the subjects did not know which supplements they were receiving. Each morning at home, subjects orally ingested one of the assigned tablets. DALDA QUESTIONNAIRE The subjects were given a folder with their name on it as well as a subject number. In the folder were 35 response sheets and a self-survey inventory question sheet known as the Daily Analysis of Life's Demands for Athletes (DALDA). The DALDA inventory consists of 34 questions that self-report a variety of symptoms of both physical and mental stress (39). While the subjects completed the entire questionnaire each time, responses to questions #15 (sore throat), #20 (chest congestion), and #25 (running nose) only were analyzed. Subjects completed the questionnaire every other day for 10 weeks with a total of 35 visits. All three symptoms that were analyzed are physical signs of URTI. SUBJECT PHYSICAL TRAINING During the 10 week study period, all twenty athletes trained with the same endurance-based dosage protocol at a strict self-governed intensity. The training model used was 40 miles of outdoor winter road training each week. The subjects all trained at 65-70% of their individual VO2 max pace intensities (aerobic threshold). They all ran seven days per week and covered the same distance each day. All subjects maintained a self-log of their daily mileage which was submitted at the completion of the study. STATISTICAL ANALYSIS All data were analyzed using numerical discrete analysis with significance ascribed for p Zinc supplemented subjects did not show a significantly different number of days expressing symptoms of URTI symptoms compared with the control. In response to the survey prompt concerning a sore throat (Table 1), the experimental group subjects had a mean of 3.0 days/person of symptom expression, while the control group showed a mean of 4.7 days/person of symptom expression (p=.46, x2=1.55). Responses to the chest congestion symptom prompt (Table 2) showed the experimental group subjects had a mean of 1.2 days/person of symptom expression, while the control group showed a mean of 3.8 days/ person of symptom expression (p=.40, x2=1.83). Regarding the running nose symptom prompt (Table 3), the experimental group had a mean of 1.3 days/ person of symptom expression, while the control group showed a mean of 5.0 days/person of symptom expression (p=.20, x2=3.17). None of these were statistically significant.
Two of the subjects in the control group were impacted by relatively severe episodes of undiagnosed URTI resulting in a high number of days expressing URTI symptoms, ultimately resulting in lost training days. One of the subjects showed 11 days of expression of a sore throat (mean=4.7 days), 11 days expression of chest congestion (mean=3.8 days) and 12 days expression of a running nose (mean=5.0 days). The other subject had 10 days of expression on a sore throat (mean=4.7 days), 15 days expression of chest congestion (mean=3.8 days), and 17 days expression of a running nose (mean=5.0 days). DISCUSSION Aerobic exercise has been shown to increase the incidence of URTI when weekly training volume dosage exceeds approximately 20 miles per week (28). As training volume increases into the elite athlete category (aerobic elite is defined as athletes capable of >30 miles per week volume) the incidence of URTI increases significantly (28). The current study examined subjects in the 40 mile per week training volume. Any expression of URTI symptoms compromises aerobic fitness training, and in many cases leads to cessation of training for a time until the symptoms diminish. A halt in fitness training influences later competitive success for the athlete (38). It is thus important to the health and fitness of the athlete to avoid URTI, minimize the effects of URTI if symptoms are expressed, and shorten the duration of the URTI. A robust immune system is necessary to minimize the deleterious effects of URTI resulting from the infection (26). The effect of aerobic training volumes greater then 20 miles per week compromises the human immune system to a linear degree (28). During the course of the 10 week treatment period, subjects in the treatment group reported slightly fewer URTI symptoms, better overall health, and fewer affected training days compared to placebo subjects. The difference, however, was not statistically significant. Elite runners whose athletic activities cause significant physical stress are more susceptible to URTI (30). A previous study reported that nutritional supplementation can modulate the health status of these high-performance athletes (43). In this study on over the counter mineral zinc supplements, a slightly reduced incidence of URTI symptoms concerning respiratory health as measured by the DALDA assessment were reported. Zinc supplemented subjects reported both fewer URTI manifestations and had fewer affected days. The URTI symptoms reported by subjects are typical of cold and flu manifestations, and analogous to symptoms reported in other studies (33). In this current study, a total of URTI symptoms were summed by subject and included three different URTI manifestations. Well-established and clinically valid techniques (DALDA survey, URTI symptoms) were used during the course of this study. Therefore, the results of the three self-reported respiratory symptoms are valid and can be used as the groundwork for future studies on respiratory health status. Physical and psychological factors of subjects undergoing stressful situations are reported to increase the incidence of URTI (22). Overall, the subjects on 15 mg mineral zinc tablets reported slightly better respiratory health than those in the placebo group. Mineral zinc supplementation improves immune function in a variety of animal models (19). From helping to prevent anthrax infection and mortality in mice (19) to reduced duration and severity of cold manifestations in humans (35), zinc supplementation has been shown to be effective. However, zinc is not the only supplement that may help endurance athletes. Vitamin C supplementation in ultra marathoners reduced the duration and severity, but not incidence, of URTI when taken 21 days before an ultra-marathon footrace of 90 km (43). The current study did not separate URTI duration and severity from symptom incidence. Moreover, the current study did not include a controlled or inventoried diet assessment of the subjects. Since both the zinc supplemented and placebo subjects were running 40 miles per week in training volume it was thought that like many of the elite running level athletes previously studied, their diet was high in carbohydrates and low in protein intake and thus were candidates for dietary zinc deficiency (10). ACKNOWLEDGEMENTS This study was made possible by the willing participation of the entire Stillwater (MN) High School Boys Cross Country team. The authors thank Dr. Kevin Bjork, who provided medical expertise regarding oral supplementation, and to Andrew Weaver, who helped maintain the paperwork of the self-assessment inventory. REFERENCES Bach J. The multi faceted zinc dependency of the immune system. Immu Tod 1981; 4:225-227. Bishop N, Blannin A, Walsh N, Robson P, and Gleason M. Nutritional aspects of immuno-suppression in athletes. Spor Med. 1999; 28(3):151-176. Briefel R, Bialostosky K, Kennedy-Stephenson I, McDowell M, Ervin R, and Wright J. Zinc intake of the US population: findings from the third National Health and Nutrition Survey 1988-1994. J Nutri. 2000; 130:1367-73. Chandra K Excessive intake of zinc impairs immune response. J Am Med Assoc. 1984; 252:1443-46. Clarkson P. Micronutrients and exercise: anti-oxidants and minerals. I Spor Sci. 1995;13(1):26-30. Cordova A and Navas F. Effect of train-ing on zinc metabolism: changes in serum and sweat zinc concentrations in sportsmen. An Nutri Metab. 1998;42:274-282. Dardenne M, Pleau I, Nabarra B, Lafrancier P, Derrien M, Choay M, and Bach J. Contribution of zinc and other metals to the biological activity of the serum thymic factor. Proc Nat Acad Sci. USA. 1992;79:370-375. Dardenne M. Zinc and immune function Euro I Clin Nutri. 1992;56(3):20-25. Favier A. The role of zinc in reproduction. Bio Tr Elem Res. 1992; 32:363-382. Fogelholm M, Laasko J, Lehto J, and Ruokonen B. Dietary intake and indicators of magnesium and zinc status in male athletes. Nutri Res. 1991; 11(10)3 111-18. Fraker P, King L, Garvey B, and Medina C. Immunopathology of zinc deficiency: a code for apoptosis. Hu Nutri. 1993;38(67):267-283. Fraker P, King L, Laako T, and Vollmer T. The dynamic link between the integrity of the immune system and zinc status. I of Nutri 2000;130:1399-1406. Frieke C. Function and mechanism of zinc. J Nutri. 2000;130:1437-65. Gleason M and Bishop N. Elite athlete immunology: importance of nutrition. Int J Spor Med. 2000;21(1):44-50. Gleason M, Nieman D and Pederson B. Exercise, nutrition and immune function. J Spor Sci. 2004;22(1):115-25. Hadden J. Thymic endocrinology. IntJ Immunophar. 1992;14:345-352. Hawley J, Dennis S, Lindsay F, and Noakes T. Nutritional practices of athletes: are they sub-optimal? J Spor Sci. 1995;13(1):75-81. Heath G, Ford F, Craven T, Macera C, Jackson K, and Pate R. Exercise and the incidence of upper respiratory tract infections. Med Sci Spor Exer. 1991;23(2):152157. Ibs K and Rink L. Zinc-altered immune function. J Nutri. 2003;133(1):1452-56. Keen C and Gershwin M. Zinc deficiency and immune function. Ann Rev Nutri. 1990;10:415-431. Killic M, Kasim A and Gunnay M Effect of zinc supplementation on hematological parameters in athletes. Bio Tr Ele Res 2004;100(1):31-38. Kohut M, Boehm G and Moynihan J. Prolonged exercise suppresses antigen specific cytokine response to upper respiratory tract infection. JApp Phys. 2001;90(2) 678-84. Lesourd B. Nutrition and immunity in the elderly: modification of immune responses with nutritional treatments. American I Clin Nutri. 1997;66:478-484. Lukaski H. Magnesiun, zinc, and chromium nutrition and athletic performance. Am J Clin Nutri. 2000;72:85-93. Lukaski H. Micronutrients (magnesium, zinc, and copper): are mineral supplements needed for athletes? Intl Spor Nutri. 1995;5(6):74-83. Lukaski H, Hoverson B, Gallagher S, and Bolonchuk W. Physical training and copper, iron, and zinc status of swimmers. The Am J Clin Nutri. 1995;51(6)3093-99. Mackinnon L and Hooper S. Mucosal immune responses to exercise of varying intensity and during overtraining. IntJ Spor Med. 1995;15:179-183. Martin S, Pierce B and Woods J. Exercise and respiratory tract viral infections. Exer Spor Sci Rev. 2009;37(4):157-164. Mertz W. Risk assessment of essential trace elements: new approaches to assessing recommended dietary allowances and safety limits. Nutri Rev. 1995;53:179-185. Nieman D. Exercise Immunology: future directions for research related to athletes, nutrition, and the elderly. Int J Med. 2000a;21(1):61-68. Nieman D. Is infection linked to exercise workload? Med Sci Spor Exerc. 2000b;32(7):406-411. Nieman D. Immune response to heavy exertion. JApp Phys. 1997a;82(5):1385-94. Nieman D. Risk of upper respiratory tract infection in athletes: an epidemiologic and immunologic perspective. J Ath Train. 1997b;32(4):344-49. Nieman D, Johanssen L and Lee J. Infectious episodes in runners before and after a running roadrace. I Spor Med Phys Fit. 1989;29(3):289-296. Peake J, Gerrard D and Griiffin J. Plasma zinc and immune markers in runners in response to a moderate increase in training volume. Int J Spor Med. 2003;24(3):212-216. Prasad A. Zinc: the biology and therapeutics of an ion. Ann Intern Med. 1996;125(2):142-148. Rink L. and Gabriel P. Extracellular and immunological actions of zinc. Biome. 2001;4:367-383. Robson Ansley P, Gleason M and Ansley L. Fatigue management in the preparation of Olympic athletes. J Spor Sci. 2009;27(13):1409-20. Rushall B. A tool for measuring stress tolerance in elite athletes. App Spor Psy. 1990;(2):51-66. Sandstead M. Zinc requirement and the risk benefit of zinc supplementation. I Tra Ele Med Bio. 2006; 20(1):3-18. Shanker A and Prasad A. Zinc and immune function: the biological basis of altered resistance to infections. Am J Clin Nutri. 1998;68(2):447-463. Singh A, Faillla M and Deuster P. Exercise-induced changes in immune function: effects of zinc supplementation. I App Phys. 1994;76(6):2298-2303. Talbott S and Talbott J. Effect of BETA 1, 3/1, 6 GLUCAN on upper respiratory tract infection symptoms and mood state in marathon athletes. J Spor Med and Sci. 2009;45(8):509-515. Vallee B. and Falchuk K The biochemical basis of zinc physiology. Phys Rev. 1993;73:79-118. Vaughan, R. Hormonal stress on elite endurance runners. Spor Med. 1998:28(4):247-261. Venkatraman J and Pendergrast D. Effects of dietary intake on immune function in athletes. Spor Med. 2002;32(5):323337. Walsh C, Sandstead H, Prasad A, Newberne P, and Fraker P. Zinc health effects and research priorities for the 1990's. Env Heal Pers. 1994;102:5-46. Wellinghausen N. Immunobiology of gestational zinc deficiency. Brit J Nutri. 2001;85(2):81-86. Williams, M. Dietary supplements and sports performance: minerals. I Int Soc Spor Nutri. 2005; 2(1):43-49. BIOS: Scott Christensen has been the Boys Cross Country and Track & Field Coach at Stillwater High School (MN) for over 30 years. He currently heads up the Track& Field Academy's Endurance program and serves as the program's lead instructor. Dr. Janet E. Steele is a professor in the Department of Biology at the University of Nebraska at Kearney. |