|
IRON - The Missing Nutritional Link to Performance by Rebecca J. Gusmer, Donald R. Dengel, PH.D. Originally Published in Techniques Magazine - Official Publication of the
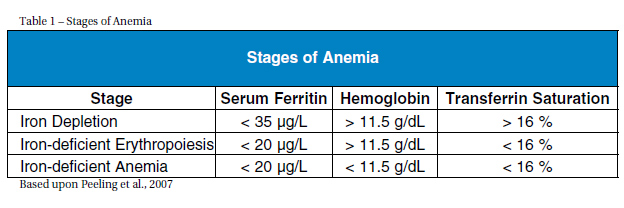
IMPORTANCE The prevalence of low iron levels is greater among athletes than the general population (Koehler et al., 2011). For instance, in a study of 193 elite athletes (96 males, 97 females) with a mean age of 16.2 +/- 2.7, iron depletion occurred among 31 percent of the males and 57 percent of the females (Koehler et al., 2011). Although iron deficiency occurs in both male and females, it most frequently occurs in female athletes, affecting about 60 percent of female athletes (Cowell et al., 2003). IRON PHYSIOLOGY The percentage of the blood volume that is made up of formed elements is termed hematocrit and reflects erythrocyte concentrations. Normal ranges of hematocrit are 42-52 percent in males and 37-47 percent in females (Pagana & Pagana, 2010). Optimal facilitation of oxygen transport requires having low to normal levels of hematocrit and slightly increased numbers of erythrocytes (Kenny, Wilmore & Costill, 2011). A low hematocrit is often seen in endurance runners but is due more to an increase in plasma volume rather than low erythrocyte production (e.g, hemodilation) and is a reason why making an accurate diagnosis of iron deficiency difficult when solely using hemoglobin levels in athletes (Kenny, Wilmore & Costa 2011). Under normal conditions, dietary intake provides the necessary amount of iron which is absorbed in the intestines (Papanikolaou & Pantopoullos, 2004). Once iron is absorbed, about 95 percent of it is bound to transferrin which transports iron to the bone marrow for production of erythrocytes or to the liver for storage as ferritin (Chatared et al., 1999). Healthy individuals store 3-5 g of iron within hemoglobin, myoglobin (a protein similar to hemoglobin that transports oxygen in the muscles) and enzymes in the liver, spleen, and bone marrow (Papanikolaou & Pantopoullos, 2004). Anemia is precipitated by iron deficiency and occurs when there are too few red blood cells or hemoglobin; its hallmark sign is exhaustion (Eichner, 2001). The three stages of anemia are iron depletion (marked by depleted iron stores), iron-deficient erythropoiesis (marked by diminished erythrocyte production) and reduced marrow supply and iron-deficient anemia (characterized by falling hemoglobin levels) (Table 1) (Peeling et al., 2007).
Diet. Inadequate intake of iron is considered the most common cause among female athletes and non-athletes (Cowell et al., 2003). The recommended dietary allowances (RDA) for women ages 19-50 is 18 mg/day, and for men ages 19-50 is 8 mg/day (Trumbo et al., 2001). Low caloric diets that are high in carbohydrates and low in animal protein and fat account for the greatest risk for developing iron deficiency (Ryan, 2004). Female athletes are at greater risk for iron deficiency since they commonly have lower energy intakes but have a higher iron requirement than males (Chatard et al., 1999). Strenuous Training. Training accelerates hemolysis, or destruction of red blood cells, as a result of the mechanical trauma associated with repeated foot strikes. This hemolysis is responsible for decreased hemoglobin levels since hemoglobin is lost in the urine when significant hemolysis occurs (Chatard et al, 1999). Furthermore, when training is too frequent, the low hemoglobin levels become permanent. The physiological effect of training on iron stores involves saturation in transferrin, the carrier that transports iron (Chatard et al., 1999). This saturation halts iron release from intestinal mucosal cells. Therefore, when transferrin saturation is high, intestinal absorption of dietary iron is decreased. This effect indicates why rest is recommended as treatment for individuals experiencing sports anemia (Chatard et al., 1999). Sweating. Sweat contains about 300 to 400 μg of iron per liter of sweat (Chatard et al., 1999). A sweat rate of 2 to 3 L per hour can result in a loss of 1 to 2 mg of iron (Chatard et al., 1999). These losses are highly individualistic and vary between body sites. Additionally, during a long distance run, sweat rates are higher at the beginning than at the end (Kenny, Wilmore & Costa 2011). Blood Loss. Blood loss is another contributor of decreased iron levels. A negative iron balance can occur with a daily blood loss of 7 to 10 mL (Chatard et al., 1999). Gastrointestinal bleeding (GI) from vigorous training typically goes unnoticed and does not have pathological consequences. Factors that affect GI bleeding include exercise intensity and distance, dehydration level and ingestion of pharmacological agents. Blood loss can also be detected microscopically in the stool or urine, with the latter being termed hematuria. About 1 to 2 percent of runners are affected by blood in the stool (Chatard et al., 1999). This unnoticed blood loss can result in a loss of 1-7 mL of blood/day, which is equivalent to about 0.5-2 mg of iron/day (Chatard et al., 1999). The causes of red blood cells found in the urine include foot- strike hemolysis, kidney damage, anti-inflammatory drug use, dehydration, and muscle tissue damage (Chatard et al., 1999). As a means to minimize internal bleeding, recommendations include maintaining adequate hydration prior to, during and after training. Menstruation. Females are at an increased risk for iron deficiency because of menstruation, which can result in a loss of 30 mL of blood per menstrual cycle (Chatard et al., 1999). This loss is equivalent to 0.5-0.6 mg of iron per day during the menstrual cycle (Chatard et al., 1999). The menstrual flow is inversely associated with serum ferritin levels. An increased flow results in a decrease in serum ferritin levels. All of these factors can result in suboptimal iron levels in athletes who engage in intense physical training. The result of this suboptimal iron level would be a reduction in performance. For this reason alone maintaining healthy iron levels is desired in athletes.
To determine an athlete's iron status, a blood sample must be drawn. A blood-testing battery including serum ferritin, serum iron, hemoglobin, transferrin, and percent transferrin saturation is desired (Fallon, 2004). However, if resources are limited, the recommendation is to check serum ferritin levels (Worwood, 1996). These levels are directly proportional to total iron stores such that every 1 μg of ferritin is equivalent to 8 mg of iron storage (Walters, 1973). Using only hemoglobin saturation levels is not recommended because they can be affected by plasma volume expansion, which frequently occurs in endurance athletes (Chatard et al., 1999). Additionally, serum iron levels have hour-to-hour variations, displaying a peak in the morning and lull in the evening, so using serum iron levels alone is not recommended for diagnosis (Worwood, 1997; Chatard et al., 1999). Standardizing testing by conducting tests at the same time of the day, without prior workouts, and without recent ingestion of iron is important for accurate results (Pagana & Pagana, 2009).
Athletes in training also make the interpretation of diagnostic levels difficult because training affects the iron parameters. For instance, hematocrits between 40-42 percent can occur without a decrease in circulating hemoglobin in endurance athletes (Chatard et al., 1999). Additionally, hemoglobin levels in endurance-trained athletes are commonly below the average population's hemoglobin ranges (i.e., 13-14 g/dL in males and 12g/dL in females) (Chatard et al., 1999). The general diagnostic levels that indicate supplementation are serum ferritin levels between 30-35 μg/L, with levels greater than 40 μg/L requiring no action (Chatard et al., 1999; Fallon, 2004; Nielsen & Nachtigall, 1998). Supplementation is recommended when transferrin saturation levels fa below 16 percent because red cell production needs cannot be met (Chatard et al., 1999). Hemoglobin values below 12 g/dL, combined with low serum ferritin levels, are additional indicators for supplementation (Pagana & Paganan, 2009; Fallon, 2004).
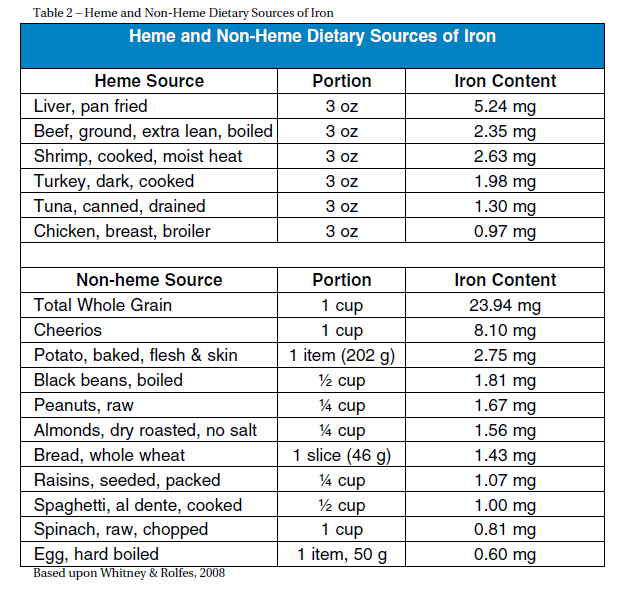
PREVENTION Dietary sources of iron are classified into heme and nonheme sources (Table 2). Foods with heme iron are more desired since 10-30 percent of the iron is absorbed in contrast to non-heme sources of which only 2-10 percent of iron is absorbed (Ryan, 2004). Heme iron is found in animal sources including lean red meat, dark poultry, and liver. Shiraki et al. found that consuming 2 g of animal protein per kg of body- weight is recommended to prevent an iron deficit (as cited in Charatard et al., 1999). For a 150-pound person this is equivalent to about five ounces of meat per day. Non-heme sources of iron are found in plants such as dark green leafy vegetables, nuts, dried fruit, beans, and iron fortified cereals (Chartard et al., 1999). To optimize dietary intake of iron, one should consume small amounts of iron rich meat multiple times a week. Heme and non-heme sources should be consumed together. To ensure maximal absorption, iron should be consumed with Vitamin C sources such as tomatoes, citrus fruits, bell peppers and spinach (Ryan, 2004). Iron absorption can be inhibited by various sources. These include calcium, phosphates, phytates (cereal grains), bran, polyphenols (tea and coffee) and antacids (Ryan, 2004). Medications such as antibiotics as well as magnesium, aluminum, calcium salts, copper, zinc and oxides can interfere with iron absorption (Chatard et al., 1999). Subsequently, intake of these sources should be avoided at the same time of consumption of iron rich foods.
Iron supplementation is provided as oral treatment through a pill (most common), as a liquid and by intramuscular injections (Chatard et al., 1999). The most common oral forms include ferrous fumarate, ferrous gluconate and ferrous sulfate which contain 33, 12 and 20 percent of elemental iron (iron available for absorption) respectively and ferric iron (Skidmore-Roth, 2010). According to Nielsen et al. (Nielsen & Nachtigall, 1998), ferrous iron salts are recommended over ferric iron because ferric iron has lower bioavailability. Among NCAA Division I institutions that provide iron supplements, the most prevalent supplemental dose is > 300 mg (>60 mg elemental) of ferrous sulfate/day (Cowell et al., 2003). However, Beard and Tobin (2000) noted that 125 mg ferrous sulfate/day is sufficient to maintain serum ferritin levels in competitive athletes. Eichner (2001) recommends 325 mg ferrous sulfate/day for individuals with serum ferritin values < 20 μ/L. Conclusively, the recommendation for individuals indicated for iron supplementation (serum ferritin < 35 μg/L) is ferrous sulfate with total doses between 125-325 mg/day for supplementation (Table 3). Individuals taking iron supplementation should be educated on the proper way to optimize absorption. This includes consuming iron with Vitamin C, taking the supplement at the same time daily (and two hours apart from other medications) and avoiding taking iron with foods that inhibit absorption (calcium, caffeine). Additionally, the individual should be encouraged to eat red meat and be provided with information on iron-rich foods and foods that inhibit iron absorption. Clinical and laboratory criteria are used to determine the effectiveness of iron supplementation. For instance, decreased fatigue, increased physical performance, ferritin levels, hemoglobin levels, transferrin saturation and appearance of reticulocytes are all indicative of effective treatment (Chatard et al., 1999). Hemoglobin should increase about 1 g/dL per week as hemoglobin levels typically increase proportionally to increases in iron supplementation (Ryan, 2004). Caution should be taken when analyzing an athlete's hemoglobin and hematocrit levels because of the hemodilution that occurs in athletes due to training resulting in significant variation. Typically, full iron repletion requires three months of oral supplementation but the length of supplementation is dependent on the individual (Peeling et al., 2007; Cowell, 2003). Consequently, follow-up testing is strongly encouraged every six months (Nielson & Nachitgall, 1998). Gary Wilson, the Head Women's Cross Country Coach at the University of Minnesota, has integrated iron testing into his coaching program for 25 years. He notes that at least three or four tests per year are necessary to get a normal level for each individual, because what might be normal for one person may be detrimentally low for another. One athlete's normal serum ferritin levels may fluctuate between 80-85 μg/L but another's may fluctuate between 40-45 μg/L even though both athletes may be at their optimal levels.
Increased risk of effects from iron toxicity occurs when serum ferritin levels are greater than 200 μg/L, which is considerably higher than individuals indicated for iron supplementation with serum ferritin levels less 35 μg/L (Chatared et al., 1999; Nielsen & Nachiticall, 1998). To safeguard against any risks, iron supplementation should not be initiated without first determining one's iron levels, and a physician should be consulted when therapeutic doses are provided (Akabas & Dolins, 2005; Ryan, 2004). The excess iron has a potential to cause dysfunction in the brain, liver and heart (Pagana & Pagana, 2009). A rare condition termed hemochromatosis occurs when an individual absorbs two to three times more iron from their diet than individuals without hemo-chromatosis (Ryan, 2004). These individuals are at risk for liver and intestinal damage if they receive iron supplementation. This situation is a rare phenomenon because individuals with hemochromatosis are rarely iron deficient.
• Serum ferritin levels below 35 μg/L are suggested to be supplemented with 125-325 mg ferrous sulfate/day (Nielsen & Nachigall, 1998; Cowell et al., 2003; Eichner, 2001) • Iron should be consumed with Vitamin C and apart from calcium and caffeine (Ryan, 2004) • Iron testing should be done 3-4 times/year to determine the normal iron levels for each athlete and monitored consistently though seasons • Hemoglobin saturation levels used solely for diagnosis are not recommend due to hemodilution (Chatard et al., 1999) • Serum iron levels used solely are not recommended due to hourly variations (Worwood, 1997; Chatard et al., 1999) • Effects from iron toxicity can occur when serum ferritin >200 μg/L and is uncommon for individuals indicated for iron deficiency (serum ferritin < 35 μg/L) (Ryan, 2004; Nielsen & Nachtigall, 1998) Conclusively, monitoring the iron status of athletes may be the missing nutritional link for optimal performance.
Auersperger, I., Knap, B., Jerin, A., Blagus, R., Lainscak, M., Skitek, M., & Skof, B. (2012). Effects of 8 weeks of endurance running on hepcidin concentrations, inflammatory parameters, and iron status in female runners. International Journal of Sport Nutrition and Exercise Metabolism, 22, 55-63. Beard, J. & Tobin, B. (2000). Iron status and exercise. The American Journal of Clinical Nutrition, 72(Suppl.), 594S-597S. Chatard, J., Mujika, I., Guy, C., & Lacour, J. (1999). Anaemia and iron deficiency in athletes: Practical recommendations for treatment. Journal of Sports Medicine, 27(4), 229-240. Coleman, D. H., Stevens, A. R., Dodge, H. T., & Finch, C. A. (1953). Rate of blood regeneration after blood loss. Archives of Internal Medicine, 93, 341-349. Cowell, B. S., Rosenbloom, C. A., Skinner, R., & Summers, S. H. (2003). Policies on screening female athletes for iron deficiency in NCAA division-I-A institutions. International Journal of Sport Nutrition and Exercise Metabolism, 13, 277- 285. Eichner, R. (2001). Anemia and blood boosting. Sports Science Exchange #81, 2(14), 81-84. Fallon, K. E. (2008). Screening for haematological and iron- related abnormalities in elite athletes-analysis of 576 cases. Journal of Science and Medicine in Sport, 11, 329-336. Fallon, K. E. (2004). Utility of hematological and iron- related screening in elite athletes. Clinical Journal of Sports Medicine, 14(3), 145-152. Gardner, G. W., Edgerton, V. R., Senewiratne, B., Barnard, R. J., & Ohira, Y. (1977). Physical work capacity and metabolic stress in subjects with iron deficiency anemia. American Journal of Clinical Nutrition, 30, 910-917. Kenny, W. L., Wilmore, J. H., & Costa D. L. (2012). Physiology of sport and exercise (5th ed.). Champagne, IL: Human Kinetics. Koehler, K., Braun, H., Achtzehn, S., Hildebrand, U., Predel, H., Mester, J., & Schanzer,W. (2011). Iron status in elite young athletes: gender-dependent influences of diet and exercise. European Journal of Applied Physiology, 112, 513-523. Lampe, J. W., Slavin, J. L., & Apple, F. S. (1991). Iron status of active women and the effect of running a marathon on bowel function and gastrointestinal blood loss. International Journal of Sports Medicine, 12, 173-179. Leggett, B. A., Brown, N. N., Byrant, S. J., Duplock, L., Powell, L. W., Halliday, J. W. (1990). Factors affecting theconcentrations of ferritin in serum in a healthy Australian population. Clinical Chemistry, 36(7), 135-1355. Milic, R., Martinovic, J., Dopsaj, M., & Dopsaj, V. (2011). Haematological and iron-related parameters in male and female athletes according to different metabolic energy demands. European Journal of Applied Physiology, 111, 449- 458. Nielsen, P. & Nachtigall, D. (1998). Iron supplementation in athletes: Current recommendations. Journal of Sports Medicine, 26(4), 207-216. Nieman, D. C. (1988). Vegetarian dietary practices and endurance performance. The American Journal of Clinical Nutrition, 48,745-761. Pagana, K. D. & Pagana, T. J. (2009). Mosby's diagnostic and laboratory test reference (9th ed.). Williamsport, PA: Elsevier Inc. Papanikolaou, G. & Pantopoulos, K. (2004). Iron metabolism and toxicity. Toxicology and Applied Pharmacology, 202(2), 199-211. Peeling, P., Bleep T., Goodman, C., Dawson, B., Claydon, G., Beilby, J., & Prins, A. (2007). Effects of iron injections on aerobic-exercise performance of iron-depleted female athletes. International Journal of Sport Nutrition and Exercise Metabolism, 17, 221-231. Ryan, M. (2004). Preventing and treating iron deficiency in athletes. Athletic Therapy Today, 9(2), 56-57. Skidmore-Roth, L. (2010). Mosby's nursing drug reference (23rd ed). St. Louis, MO: Elsevier Inc. Telford, R. D. & Cunningham, R. B. (1991). Sex, sport, and body-size dependence of hematology in highly trained athletes. Medicine and Science in Sports and Exercise, 23(7), 788-794. Trumbo, P., Yates, A. A., Schlicker, S., & Poos, M. (2001). Dietary reference intake: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Journal of the American Dietetic Association, 101(3), 294-301. Walters, G. 0., Miller, F. M., & Worwood, M. (1973). Serum ferritin concentration and iron stores in normal subjects. Journal of Clinical Pathology, 26, 770-772. Whitney, E. & Rolfes, S. R. (2008). Understanding nutrition. Mason, OH: Thomson Wadsworth Widmaier, E. P., Raff, H., Strang, K. T. (2008). Vander's human physiology: The mechanisms of body function (11th ed.). New York, NY: McGraw-Hill Companies, Inc. Worwood, M. (1997). Review article: The laboratory assess-ment of iron status - an update. Clinical Chimica Acta 259, 2-23. Rebecca Gusmer is a cross country and track and field athlete at the University of Minnesota majoring in kinesiology. Donald R. Dengel, Ph.D., is an Associate Professor in the School of Kinesiology and Director of the Human Performance Core and Densitometry Services in Clinical and Translational Science Institute at the University of Minnesota. |
|
|